Are you trying to understand the critical role of fibroblasts in connective tissue and how they impact your health? At rental-server.net, we’ll guide you through the functions of these essential cells and explore how they contribute to tissue repair and overall well-being. Discover how fibroblasts maintain the structural integrity of your organs with robust dedicated server solutions and connective tissues, ensuring optimal function and resilience, alongside innovative VPS hosting.
Table of Contents
- 1. What Are Fibroblasts and Connective Tissue?
- 2. Key Functions of Fibroblasts in Connective Tissue
- 3. How Do Fibroblasts Contribute to Wound Healing?
- 4. Fibroblasts and the Extracellular Matrix (ECM)
- 5. The Role of Myofibroblasts in Tissue Repair
- 6. Fibroblast Heterogeneity Across Different Tissues
- 7. Signaling Pathways Regulating Fibroblast Function
- 8. Fibroblasts in Skin: Structure, Function, and Repair
- 9. Lung Fibroblasts: Maintaining Elasticity and Gas Exchange
- 10. Skeletal Muscle Fibroblasts: Supporting Muscle Regeneration
- 11. Cardiac Fibroblasts: Ensuring Heart Muscle Integrity
- 12. Fibroblasts and Fibrosis: What Happens When Things Go Wrong?
- 13. Emerging Therapies Targeting Fibroblasts
- 14. FAQ: Understanding Fibroblast Functions
1. What Are Fibroblasts and Connective Tissue?
Are you curious about the basic components of your body that keep everything structurally sound? Fibroblasts are cells within connective tissue that play a crucial role in maintaining its integrity and function. Connective tissue, found throughout the body, provides support, structure, and connection for other tissues and organs.
Fibroblasts, described as “spindle-shaped cells of the connective tissue” by Rudolf Virchow in 1858, synthesize and maintain the extracellular matrix (ECM), a complex network of proteins and molecules that surrounds and supports cells, similar to how rental-server.net provides robust IT infrastructure. Ernst Ziegler later coined the term “fibroblast” to describe cells that produce new connective tissue during healing. Connective tissues include bone, cartilage, adipose tissue, blood, and fibrous tissues, each with distinct functions supported by specialized ECM, which is crucial to robust bare metal server hosting.
1.1 Types of Connective Tissue
Connective tissue is categorized into several types based on its structure and function:
- Loose Connective Tissue: Supports and cushions organs.
- Dense Connective Tissue: Provides strength and support to tendons and ligaments.
- Cartilage: Provides flexible support in joints and other areas.
- Bone: Provides rigid support and protection.
- Blood: Transports nutrients and waste products.
1.2 The Role of Connective Tissue in the Body
Connective tissue performs diverse functions, including:
- Providing structural support for organs and tissues.
- Connecting different tissues and organs.
- Protecting organs from damage.
- Facilitating nutrient and waste exchange.
- Aiding in wound healing and tissue repair.
The health and functionality of connective tissue are critical for overall bodily function, much like the reliable uptime provided by dedicated hosting services.
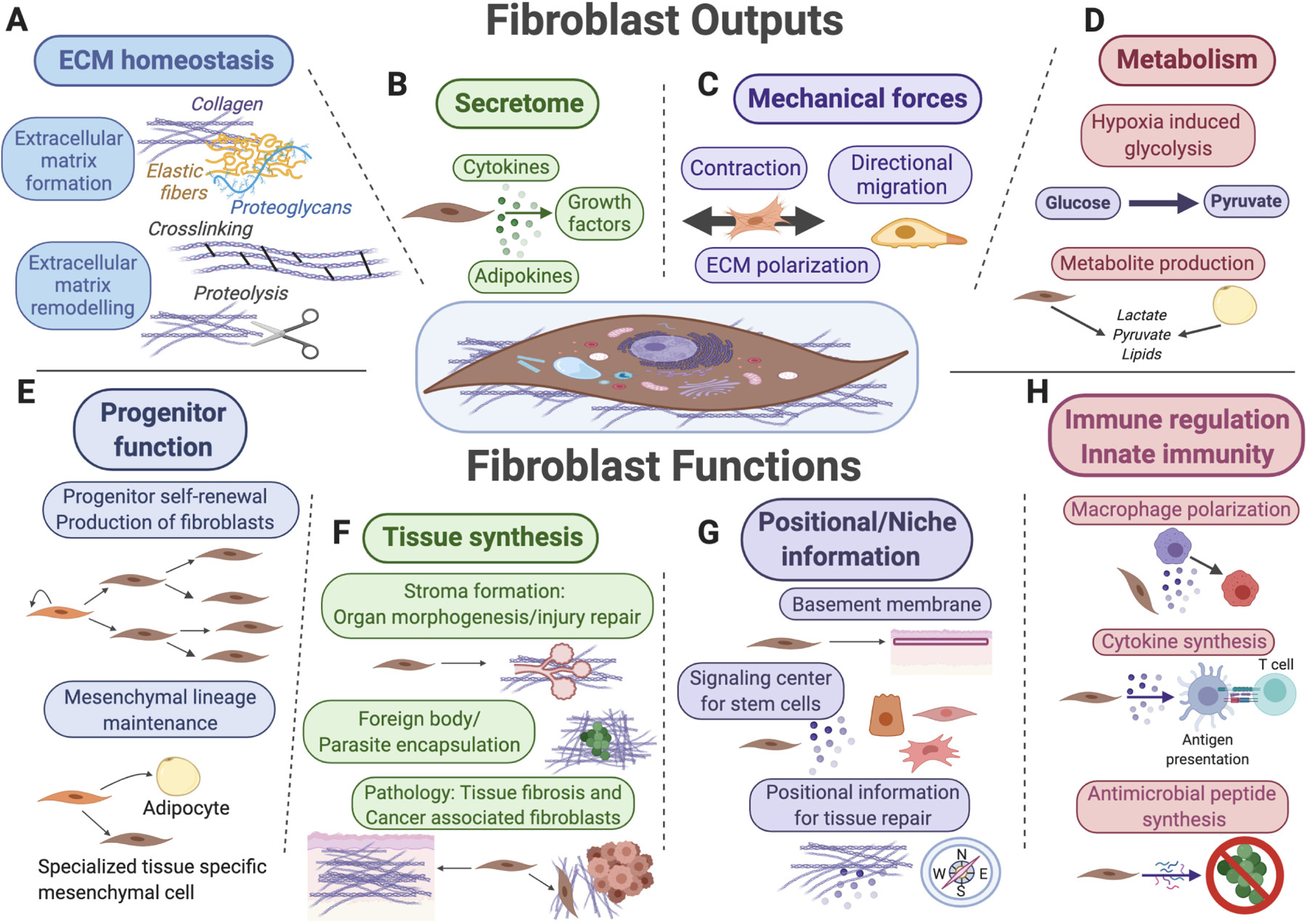 Fibroblasts in Connective Tissue
Fibroblasts in Connective Tissue
Image: Illustration showing fibroblasts in connective tissue, highlighting their role in ECM secretion and signaling. The functions of fibroblasts include ECM remodeling, secretion of signaling factors, mechanical force generation, regulation of tissue metabolism, and progenitor cell functions. This showcases their integral role in maintaining tissue homeostasis and repair.
2. Key Functions of Fibroblasts in Connective Tissue
What crucial roles do fibroblasts play in maintaining the structure and health of your tissues? Fibroblasts are responsible for several essential functions that contribute to tissue integrity and overall function, akin to how rental-server.net ensures efficient and reliable server performance.
2.1 Extracellular Matrix (ECM) Production
Do you know what the primary role of fibroblasts is? The primary function of fibroblasts is to produce and maintain the extracellular matrix (ECM), the structural framework that supports cells in tissues. This involves synthesizing and secreting various proteins, including:
- Collagen: Provides tensile strength.
- Elastin: Allows tissues to stretch and recoil.
- Fibronectin: Promotes cell adhesion and migration.
- Proteoglycans: Regulate tissue hydration and elasticity.
According to research from the National Institutes of Health, the precise composition of the ECM varies depending on the tissue type and its specific functional requirements. This variability is crucial for the specialized roles of different connective tissues.
2.2 Tissue Repair and Wound Healing
Have you ever wondered how your body repairs itself after an injury? Fibroblasts play a central role in tissue repair and wound healing, similar to the proactive support of reliable server solutions. They migrate to the site of injury, proliferate, and synthesize new ECM components to rebuild damaged tissue. This process involves several stages:
- Inflammation: Immune cells release signals that attract fibroblasts to the wound site.
- Proliferation: Fibroblasts rapidly divide and increase in number.
- Matrix Synthesis: Fibroblasts produce collagen and other ECM components to fill the wound.
- Remodeling: The newly synthesized ECM is remodeled to restore tissue structure and function.
2.3 Signaling and Communication
Did you know fibroblasts also act as communication hubs in tissues? Fibroblasts secrete various signaling molecules, such as cytokines and growth factors, that regulate the behavior of neighboring cells, mirroring the seamless communication facilitated by efficient cloud server solutions. These signaling molecules influence processes like:
- Cell Growth and Proliferation: Promoting cell division and expansion.
- Inflammation: Modulating the immune response to injury.
- Angiogenesis: Stimulating the formation of new blood vessels.
- Matrix Remodeling: Regulating the breakdown and synthesis of ECM components.
These signaling functions enable fibroblasts to coordinate tissue responses to injury and maintain tissue homeostasis.
2.4 Mechanical Force Generation
Are you aware that fibroblasts can exert mechanical forces on their surroundings? Fibroblasts can contract and exert mechanical forces on the ECM, which is essential for tissue remodeling and wound closure, similar to how scalable VPS solutions adapt to changing demands. These forces are generated through the action of contractile proteins within the fibroblast, such as alpha-smooth muscle actin (α-SMA).
Mechanical force generation by fibroblasts plays a crucial role in:
- Wound Contraction: Reducing the size of the wound and bringing the edges closer together.
- Tissue Remodeling: Aligning collagen fibers and shaping the ECM.
- Organ Morphogenesis: Guiding the development and shaping of organs during embryonic development.
 Key Functions of Fibroblasts
Key Functions of Fibroblasts
Image: Diagram showing key roles of signaling pathways in regulating fibroblast functions, including PDGF, TGFβ, and WNT signaling. These pathways regulate proliferation, self-renewal, myofibroblast formation, and differentiation, influencing various aspects of fibroblast biology and fibrosis.
3. How Do Fibroblasts Contribute to Wound Healing?
When you get a cut, what exactly is happening at the cellular level to heal the wound? Fibroblasts play a pivotal role in wound healing, orchestrating the repair process to restore tissue integrity, akin to how rental-server.net ensures swift recovery from server issues. Their contribution can be summarized in several key steps:
3.1 Initial Response to Injury
What happens immediately after an injury? Following an injury, platelets and immune cells release signaling molecules that attract fibroblasts to the wound site. These signals include growth factors and cytokines, such as:
- Platelet-Derived Growth Factor (PDGF): Stimulates fibroblast migration and proliferation.
- Transforming Growth Factor Beta (TGF-β): Promotes ECM synthesis and myofibroblast differentiation.
- Interleukin-1 (IL-1): Enhances inflammation and fibroblast activation.
According to a study in the “Journal of Cell Biology,” these signaling molecules create a chemotactic gradient that guides fibroblasts to the damaged area, ensuring that the repair process begins promptly.
3.2 Fibroblast Proliferation and Migration
How do fibroblasts multiply and move to the injury site? Once at the wound site, fibroblasts undergo rapid proliferation and migration to increase their numbers and fill the damaged area. This process is facilitated by:
- Growth Factors: Stimulate fibroblast division and expansion.
- Cell Adhesion Molecules: Enable fibroblasts to attach to the ECM and migrate along it.
- Matrix Metalloproteinases (MMPs): Enzymes that degrade the ECM, allowing fibroblasts to move through it.
The “Journal of Investigative Dermatology” highlights that fibroblasts’ ability to remodel the ECM is crucial for their migration and proliferation, allowing them to effectively repair the wound.
3.3 Collagen Synthesis and Matrix Deposition
What role does collagen play in wound repair? Fibroblasts are responsible for synthesizing and depositing collagen, the primary structural protein in the ECM. Collagen provides strength and support to the newly formed tissue, akin to the resilient and scalable architecture of cloud server solutions. The steps involved in collagen synthesis include:
- Transcription: Genes encoding collagen proteins are transcribed into mRNA.
- Translation: mRNA is translated into collagen proteins in the ribosomes.
- Post-translational Modification: Collagen proteins undergo modifications, such as hydroxylation and glycosylation.
- Secretion: Modified collagen proteins are secreted into the extracellular space, where they assemble into collagen fibers.
3.4 Wound Contraction and Remodeling
How is the wound closed and reshaped during healing? Fibroblasts differentiate into myofibroblasts, specialized cells that express α-SMA and generate contractile forces, closing the wound and remodeling the tissue. This process is essential for:
- Wound Closure: Reducing the size of the wound by pulling the edges together.
- Tissue Alignment: Orienting collagen fibers along lines of stress to improve tissue strength.
- Scar Formation: Replacing damaged tissue with a fibrous scar composed primarily of collagen.
However, excessive myofibroblast activity can lead to hypertrophic scars or keloids, which are raised, thickened scars that can cause pain and restrict movement.
Image: A visual representation of wound healing stages involving fibroblasts, inflammation, proliferation, and ECM remodeling. Fibroblasts migrate to the wound site, proliferate, and synthesize new ECM, contributing to the restoration of tissue structure and function.
4. Fibroblasts and the Extracellular Matrix (ECM)
What is the extracellular matrix, and how do fibroblasts contribute to its maintenance? The extracellular matrix (ECM) is a complex network of proteins and molecules that surrounds and supports cells in tissues, with fibroblasts playing a crucial role in its synthesis, maintenance, and remodeling, akin to how rental-server.net ensures infrastructure integrity through robust data management.
4.1 Composition of the ECM
What are the main components of the extracellular matrix? The ECM consists of various components, including:
- Collagen: Provides tensile strength and structural support.
- Elastin: Allows tissues to stretch and recoil.
- Glycosaminoglycans (GAGs): Polysaccharides that regulate tissue hydration and elasticity.
- Proteoglycans: Proteins with attached GAG chains, contributing to ECM structure and function.
- Adhesive Glycoproteins: Such as fibronectin and laminin, promote cell adhesion and migration.
According to research published in “Matrix Biology,” the specific composition of the ECM varies depending on the tissue type, reflecting its unique functional requirements.
4.2 Synthesis and Deposition of ECM Components
How do fibroblasts produce and deposit these ECM components? Fibroblasts synthesize and deposit ECM components through a highly regulated process that involves:
- Gene Expression: Genes encoding ECM proteins are transcribed and translated.
- Protein Modification: ECM proteins undergo post-translational modifications, such as glycosylation and hydroxylation.
- Secretion: Modified ECM proteins are secreted into the extracellular space.
- Assembly: ECM proteins self-assemble into complex networks and structures.
Fibroblasts secrete enzymes, such as lysyl oxidase, that crosslink collagen and elastin fibers, enhancing the ECM’s mechanical properties.
4.3 ECM Remodeling and Degradation
How is the ECM remodeled and broken down? ECM remodeling is a dynamic process involving the synthesis, degradation, and reorganization of ECM components. Fibroblasts regulate ECM remodeling through:
- Matrix Metalloproteinases (MMPs): Enzymes that degrade ECM components, allowing for tissue turnover and repair.
- Tissue Inhibitors of Metalloproteinases (TIMPs): Regulate MMP activity, preventing excessive ECM degradation.
- Crosslinking Enzymes: Such as lysyl oxidase, stabilize ECM structure by crosslinking collagen and elastin fibers.
The balance between ECM synthesis and degradation is crucial for maintaining tissue homeostasis and responding to injury.
4.4 The Role of ECM in Tissue Function
How does the ECM affect tissue function? The ECM provides structural support and regulates cell behavior, influencing processes like:
- Cell Adhesion: ECM proteins, such as fibronectin and laminin, promote cell attachment and spreading.
- Cell Migration: ECM provides a substrate for cell movement, guiding cells during development and wound healing.
- Cell Proliferation: ECM components can stimulate or inhibit cell division, regulating tissue growth and repair.
- Cell Differentiation: ECM signals can influence cell fate and differentiation, determining tissue structure and function.
Disruptions in ECM structure and function can lead to various diseases, including fibrosis, cancer, and arthritis.
Image: A detailed visual of the extracellular matrix, showcasing components like collagen fibers, proteoglycans, and adhesive glycoproteins. This network supports cells in tissues and regulates various cell behaviors through structural support and signaling.
5. The Role of Myofibroblasts in Tissue Repair
What are myofibroblasts, and how do they contribute to tissue repair? Myofibroblasts are specialized fibroblasts that play a critical role in tissue repair and wound contraction, exerting mechanical forces on the ECM, akin to the adaptable infrastructure that ensures consistent performance for dedicated server solutions.
5.1 Differentiation of Fibroblasts into Myofibroblasts
What triggers the transformation of fibroblasts into myofibroblasts? Fibroblasts differentiate into myofibroblasts in response to specific signals, including:
- Transforming Growth Factor Beta (TGF-β): A potent inducer of myofibroblast differentiation.
- Mechanical Stress: Forces exerted on the ECM can stimulate myofibroblast formation.
- Connective Tissue Growth Factor (CTGF): Promotes myofibroblast proliferation and ECM synthesis.
According to a study in the “American Journal of Pathology,” TGF-β is a primary driver of myofibroblast differentiation, leading to increased expression of α-SMA and ECM proteins.
5.2 Characteristics of Myofibroblasts
What distinguishes myofibroblasts from regular fibroblasts? Myofibroblasts are characterized by:
- Expression of α-Smooth Muscle Actin (α-SMA): A contractile protein that enables myofibroblasts to generate mechanical forces.
- Increased ECM Synthesis: Myofibroblasts produce large amounts of collagen and other ECM components.
- Formation of Focal Adhesions: Specialized structures that anchor myofibroblasts to the ECM and transmit mechanical forces.
- Secretion of Growth Factors and Cytokines: Myofibroblasts release signaling molecules that regulate tissue repair and inflammation.
5.3 Wound Contraction and Tissue Remodeling
How do myofibroblasts help close and reshape wounds? Myofibroblasts play a central role in wound contraction and tissue remodeling through:
- Generation of Contractile Forces: Myofibroblasts contract and exert mechanical forces on the ECM, pulling the edges of the wound together and reducing its size.
- ECM Deposition: Myofibroblasts synthesize and deposit collagen and other ECM components, providing structural support to the newly formed tissue.
- Matrix Alignment: Myofibroblasts align collagen fibers along lines of stress, improving tissue strength and organization.
The “Journal of Surgical Research” highlights that wound contraction mediated by myofibroblasts is essential for efficient wound closure and minimizing scar formation.
5.4 Role in Fibrosis and Scarring
Can myofibroblasts contribute to excessive scarring? While myofibroblasts are essential for wound healing, their excessive or prolonged activity can lead to fibrosis and scarring. In chronic conditions, persistent myofibroblast activity results in:
- Excessive ECM Deposition: Leading to tissue thickening and stiffness.
- Disorganized Matrix Structure: Impairing tissue function and flexibility.
- Formation of Contractures: Restricting movement and causing pain.
Targeting myofibroblast activity is a key strategy for preventing and treating fibrotic diseases, such as liver cirrhosis, pulmonary fibrosis, and scleroderma.
Image: Representation of myofibroblasts in tissue repair, showing their expression of α-smooth muscle actin (α-SMA) and generation of contractile forces. This illustrates their crucial role in wound contraction and ECM remodeling, highlighting their function in both normal healing and fibrotic conditions.
6. Fibroblast Heterogeneity Across Different Tissues
Are fibroblasts the same in every part of your body? Fibroblasts exhibit significant heterogeneity across different tissues, reflecting their specialized roles in maintaining tissue-specific structure and function, much like the tailored IT solutions rental-server.net provides.
6.1 Tissue-Specific Markers
How do we distinguish fibroblasts from different tissues? Fibroblasts express different markers depending on their location and function. These markers can be used to identify and characterize fibroblast populations in various tissues:
- Skin: CD26 (DPP4), Lrig1, and Prdm1 are expressed in papillary fibroblasts.
- Lung: Fabp1, Fabp4, and Pparg are expressed in lipofibroblasts.
- Heart: Wif1 and Dkk3 are expressed in endocardial-derived fibroblasts.
- Skeletal Muscle: Tcf4 and Gli1 are expressed in fibro-adipogenic progenitors (FAPs).
Research from “Cell Stem Cell” emphasizes that these tissue-specific markers reflect the unique developmental origins and functional requirements of fibroblasts in different organs.
6.2 Functional Differences
What are the functional differences between fibroblasts in various tissues? Fibroblasts perform different functions depending on their tissue of origin:
- Skin: Maintain the dermal structure and regulate hair follicle development.
- Lung: Support alveolar structure and facilitate gas exchange.
- Heart: Maintain cardiac ECM and regulate electrical conductivity.
- Skeletal Muscle: Support muscle regeneration and prevent fibrosis.
These functional differences highlight the adaptability and specialization of fibroblasts in maintaining tissue homeostasis.
6.3 Developmental Origins
Do fibroblasts originate from the same source during development? Fibroblasts arise from different embryonic origins, contributing to their heterogeneity:
- Paraxial Mesoderm: Gives rise to fibroblasts in the trunk and limbs.
- Lateral Plate Mesoderm: Contributes to fibroblasts in the heart and blood vessels.
- Neural Crest Mesenchyme: Generates fibroblasts in the craniofacial region.
- Epithelial-to-Mesenchymal Transition (EMT): Generates cardiac fibroblasts from epicardial and endocardial cells.
The “Developmental Cell” journal notes that these distinct developmental origins influence the functional properties and lineage potential of fibroblasts in different tissues.
6.4 Response to Injury and Disease
How do fibroblasts react differently to injuries and diseases based on their tissue location? Fibroblasts respond differently to injury and disease depending on their tissue context:
- Skin: Dermal fibroblasts contribute to wound healing and scar formation.
- Lung: Pulmonary fibroblasts mediate fibrosis in response to chronic inflammation.
- Heart: Cardiac fibroblasts promote ECM remodeling and scar formation after myocardial infarction.
- Skeletal Muscle: Muscle fibroblasts contribute to fibrosis and impaired regeneration in muscular dystrophies.
Understanding these tissue-specific responses is crucial for developing targeted therapies to prevent and treat fibrotic diseases.
Image: An illustration of fibroblast heterogeneity across different tissues, highlighting the diversity in their morphology, marker expression, and functional roles. This emphasizes the adaptability and specialization of fibroblasts in maintaining tissue-specific homeostasis.
7. Signaling Pathways Regulating Fibroblast Function
Which signaling pathways control the behavior of fibroblasts? Several signaling pathways regulate fibroblast function, influencing their proliferation, differentiation, ECM synthesis, and response to injury, akin to the reliable and efficient data transmission pathways in dedicated server networks.
7.1 Transforming Growth Factor Beta (TGF-β) Pathway
How does TGF-β affect fibroblasts? The TGF-β pathway is a critical regulator of fibroblast function, promoting:
- Myofibroblast Differentiation: Inducing the expression of α-SMA and ECM proteins.
- ECM Synthesis: Stimulating the production of collagen, fibronectin, and other ECM components.
- Inflammation: Modulating the immune response to injury and promoting fibrosis.
TGF-β ligands bind to cell-surface receptors, activating intracellular signaling cascades that lead to the transcription of target genes.
7.2 Platelet-Derived Growth Factor (PDGF) Pathway
What role does PDGF play in fibroblast function? The PDGF pathway regulates fibroblast proliferation, migration, and ECM synthesis:
- Cell Proliferation: Stimulating fibroblast division and expansion.
- Cell Migration: Promoting fibroblast movement to the site of injury.
- ECM Remodeling: Regulating the breakdown and synthesis of ECM components.
PDGF ligands bind to receptor tyrosine kinases, activating intracellular signaling pathways that promote cell growth and survival.
7.3 Wnt Signaling Pathway
How does the Wnt pathway influence fibroblasts? The Wnt pathway is involved in fibroblast fate determination and ECM production:
- Cell Fate: Directing progenitor cells into fibroblast lineages.
- ECM Synthesis: Promoting the expression of ECM genes.
- Fibrosis: Contributing to the development of fibrotic diseases.
Wnt ligands bind to cell-surface receptors, activating intracellular signaling cascades that stabilize β-catenin and promote the transcription of target genes.
7.4 Mechanotransduction Pathways
How do mechanical forces affect fibroblast behavior? Mechanotransduction pathways mediate the response of fibroblasts to mechanical cues, influencing:
- Cell Adhesion: Promoting the formation of focal adhesions and cell attachment to the ECM.
- ECM Remodeling: Regulating the alignment and organization of collagen fibers.
- Myofibroblast Differentiation: Stimulating the expression of α-SMA and contractile proteins.
These pathways involve integrins, focal adhesion kinase (FAK), and Rho GTPases, which transduce mechanical signals into biochemical responses.
Image: A detailed diagram illustrating the signaling pathways that regulate fibroblast functions. These pathways, including TGF-β, PDGF, Wnt, and mechanotransduction, influence proliferation, differentiation, ECM synthesis, and the response to injury, playing vital roles in maintaining tissue homeostasis.
8. Fibroblasts in Skin: Structure, Function, and Repair
What unique roles do fibroblasts play in your skin? Fibroblasts in the skin are essential for maintaining the structural integrity of the dermis, regulating hair follicle development, and facilitating wound healing, mirroring the critical function of reliable data centers in infrastructure support.
8.1 Dermal Structure and Composition
How do fibroblasts contribute to the skin’s structural framework? The dermis consists of two layers:
- Papillary Dermis: Contains loose connective tissue, blood vessels, and sensory receptors.
- Reticular Dermis: Contains dense connective tissue, providing strength and elasticity.
Fibroblasts synthesize and maintain the ECM in both layers, providing structural support and regulating cell behavior.
8.2 Types of Skin Fibroblasts
Are there different types of fibroblasts in the skin? Different types of fibroblasts reside in the dermis:
- Papillary Fibroblasts: Express CD26 (DPP4) and contribute to the formation of the dermal papillae.
- Reticular Fibroblasts: Express Dlk1 and contribute to the strength and elasticity of the dermis.
- Dermal Sheath Fibroblasts: Enclose hair follicles and regulate their growth cycle.
- Dermal Papilla Fibroblasts: Regulate hair follicle development and stem cell behavior.
8.3 Role in Hair Follicle Development
How do fibroblasts influence hair growth? Dermal papilla fibroblasts and dermal sheath fibroblasts regulate hair follicle development and cycling.
- Dermal Papilla Fibroblasts: Secrete growth factors and signaling molecules that stimulate hair growth.
- Dermal Sheath Fibroblasts: Provide structural support and regulate hair follicle shape.
8.4 Wound Healing in the Skin
How do fibroblasts repair skin injuries? Skin fibroblasts migrate to the wound site, proliferate, and synthesize new ECM components, akin to efficient disaster recovery solutions. They also differentiate into myofibroblasts, which contract the wound and promote tissue remodeling.
- Inflammation: Immune cells release signals that attract fibroblasts to the wound site.
- Proliferation: Fibroblasts rapidly divide and increase in number.
- Matrix Synthesis: Fibroblasts produce collagen and other ECM components to fill the wound.
- Remodeling: The newly synthesized ECM is remodeled to restore tissue structure and function.
8.5 Fibrosis and Scar Formation
What happens when skin healing goes awry? Excessive myofibroblast activity can lead to hypertrophic scars or keloids. Therapies targeting myofibroblast activity, such as TGF-β inhibitors and mechanical unloading, can reduce scar formation and promote regenerative healing.
Image: A detailed view of skin fibroblasts within the dermal layers, showing their organization and interaction with other skin structures such as hair follicles. Fibroblasts maintain the structural integrity of the dermis and play a crucial role in hair follicle development and wound repair.
9. Lung Fibroblasts: Maintaining Elasticity and Gas Exchange
How do fibroblasts in your lungs support breathing? Lung fibroblasts are essential for maintaining the elasticity of the alveolar structure and facilitating efficient gas exchange, similar to how robust bandwidth ensures smooth data transfer.
9.1 Alveolar Structure and Function
What role do fibroblasts play in the lung’s tiny air sacs? The alveoli are tiny air sacs in the lungs where gas exchange occurs. Fibroblasts support the alveolar structure by:
- Synthesizing and maintaining the ECM: Providing structural support to the alveoli.
- Regulating alveolar elasticity: Ensuring proper expansion and contraction during breathing.
- Facilitating gas exchange: Promoting close contact between alveolar epithelium and capillaries.
9.2 Types of Lung Fibroblasts
Are there different kinds of fibroblasts in the lungs? Different types of fibroblasts reside in the lung tissue:
- Interstitial Fibroblasts: Maintain the ECM and regulate alveolar structure.
- Lipofibroblasts: Store and release lipids, supporting alveolar type II cells.
- Peribronchial Fibroblasts: Surround the airways and regulate their contraction and relaxation.
9.3 Role in Lung Development
How do fibroblasts contribute to the formation of the lungs? Lung fibroblasts regulate lung development by:
- Directing branching morphogenesis: Guiding the formation of the airways.
- Supporting alveolar formation: Promoting the development of the alveoli.
- Secreting growth factors: Regulating the proliferation and differentiation of lung cells.
9.4 Lung Injury and Repair
How do fibroblasts respond to lung damage? Lung fibroblasts migrate to the site of injury, proliferate, and synthesize new ECM components. They also differentiate into myofibroblasts, which contract the damaged tissue and promote scar formation.
- Inflammation: Immune cells release signals that attract fibroblasts to the wound site.
- Proliferation: Fibroblasts rapidly divide and increase in number.
- Matrix Synthesis: Fibroblasts produce collagen and other ECM components to fill the wound.
- Remodeling: The newly synthesized ECM is remodeled to restore tissue structure and function.
9.5 Pulmonary Fibrosis
What happens when lung healing goes wrong? Excessive myofibroblast activity can lead to pulmonary fibrosis, a chronic and progressive disease characterized by:
- Excessive ECM Deposition: Leading to tissue thickening and stiffness.
- Impaired Gas Exchange: Reducing lung function and causing shortness of breath.
- Progressive Scarring: Damaging the alveolar structure and reducing lung capacity.
Therapies targeting myofibroblast activity and ECM deposition are crucial for managing pulmonary fibrosis.
Image: An illustration showing the diverse types of lung fibroblasts, including interstitial fibroblasts and lipofibroblasts, and their contribution to alveolar structure and gas exchange. Fibroblasts maintain the elasticity of the alveolar structure and play a crucial role in lung development and repair.
10. Skeletal Muscle Fibroblasts: Supporting Muscle Regeneration
How do fibroblasts aid in muscle repair and regeneration? Skeletal muscle fibroblasts support muscle fiber regeneration, regulate the formation of new blood vessels, and prevent excessive fibrosis, similar to how efficient network management supports reliable data flow.
10.1 Muscle Structure and Function
What role do fibroblasts play in the structure of muscles? Skeletal muscles are composed of muscle fibers, blood vessels, and connective tissue. Fibroblasts contribute to the muscle structure by:
- Synthesizing and maintaining the ECM: Providing structural support to the muscle fibers.
- Regulating blood vessel formation: Ensuring adequate oxygen and nutrient supply.
- Preventing excessive fibrosis: Maintaining muscle elasticity and function.
10.2 Types of Muscle Fibroblasts
Are there different types of fibroblasts in muscles? Different types of fibroblasts reside in the muscle tissue:
- Endomysial Fibroblasts: Surround individual muscle fibers and regulate their growth and function.
- Perimysial Fibroblasts: Surround groups of muscle fibers (fascicles) and provide structural support.
- Epimysial Fibroblasts: Enclose the entire muscle and regulate its interaction with surrounding tissues.
- Fibro-Adipogenic Progenitors (FAPs): Muscle-resident progenitor cells that can differentiate into fibroblasts and adipocytes.
10.3 Muscle Regeneration
How do fibroblasts help repair damaged muscles? Muscle fibroblasts support muscle regeneration by:
- Secreting growth factors: Stimulating muscle stem cell (satellite cell) activation and proliferation.
- Providing a scaffold for new muscle fibers: Facilitating the formation of new muscle tissue.
- Regulating inflammation: Modulating the immune response to injury.
10.4 Muscle Injury and Repair
What happens when muscles are injured, and how do fibroblasts help? Muscle fibroblasts migrate to the site of injury, proliferate, and synthesize new ECM components, akin to rapid deployment solutions in disaster recovery.
- Inflammation: Immune cells release signals that attract fibroblasts to the wound site.
- Proliferation: Fibroblasts rapidly divide and increase in number.
- Matrix Synthesis: Fibroblasts produce collagen and other ECM components to fill the wound.
- Remodeling: The newly synthesized ECM is remodeled to restore tissue structure and function.
10.5 Muscular Dystrophy and Fibrosis
What happens when muscle healing goes wrong? In muscular dystrophy, chronic muscle damage leads to excessive fibroblast activity and fibrosis.
- Excessive ECM Deposition: Leading to tissue thickening and stiffness.
- Impaired Muscle Regeneration: Reducing muscle function and causing weakness.
- Progressive Scarring: Damaging the muscle structure and reducing muscle mass.
Therapies targeting fibroblast activity and ECM deposition are crucial for managing muscular dystrophy and promoting muscle regeneration.
Image: An illustration showing skeletal muscle fibroblasts supporting muscle fiber regeneration, regulating blood vessel formation, and preventing excessive fibrosis. This emphasizes their crucial role in maintaining muscle health and facilitating repair after injury.
11. Cardiac Fibroblasts: Ensuring Heart Muscle Integrity
How do fibroblasts contribute to a healthy heart? Cardiac fibroblasts maintain the structural integrity of the heart muscle, regulate electrical conductivity, and facilitate the response to injury, akin to how consistent power supply ensures uninterrupted server operation.
11.1 Heart Structure and Function
What role do fibroblasts play in the heart’s structure? The heart is composed of cardiomyocytes, blood vessels, and connective tissue. Cardiac fibroblasts contribute to the heart structure by:
- Synthesizing and maintaining the ECM: Providing structural support to the cardiomyocytes.
- Regulating electrical conductivity: Ensuring proper heart rhythm.
- Facilitating the response to injury: Promoting tissue repair and preventing excessive scarring.
11.2 Types of Cardiac Fibroblasts
Are there different types of fibroblasts in the heart? Different types of fibroblasts reside in the heart tissue:
- Interstitial Fibroblasts: Surround cardiomyocytes and regulate their growth and function.
- Perivascular Fibroblasts: Surround blood vessels and regulate their contraction and relaxation.
- Epicardial Fibroblasts: Cover the outer surface of the heart and regulate its interaction with surrounding tissues.
11.3 Heart Development
How do fibroblasts help form the heart? Cardiac fibroblasts regulate heart development by:
- Promoting cardiomyocyte differentiation: Guiding the formation of heart muscle cells.
- Regulating heart valve formation: Ensuring proper valve structure and function.
