Abstract
In contemporary clinical oncology, radiolabeled octreotide analogs are at the forefront of cancer imaging and therapy. Expanding this success to other radiopeptide families has been significantly hindered by the inherent metabolic instability of peptide analogs when administered systemically. Our research explored the hypothesis that the concurrent administration of specific enzyme inhibitors in vivo could enhance peptide bioavailability, thereby improving tumor uptake. Through the simultaneous injection of phosphoramidon (PA), a neutral endopeptidase inhibitor, we observed substantial increases in circulating levels of intact somatostatin, gastrin, and bombesin radiopeptides in murine models. This resulted in a remarkable enhancement of radiopeptide accumulation in tumor xenografts within mice.
Methods: Peptide conjugates [DOTA-Ala1]SS14 (DOTA-Ala-Gly-c[Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-Ser-Cys]-OH), PanSB1 (DOTA-PEG2-dTyr-Gln-Trp-Ala-Val-βAla-His-Phe-Nle-NH2), and DOTA-MG11 (DOTA-dGlu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2) were labeled with 111In via a 20-minute heating process at an acidic pH. Metabolic stability was assessed using high-performance liquid chromatography (HPLC) to analyze blood samples collected 5 minutes post-injection of the radiopeptide alone or with PA in mice. Biodistribution studies were conducted in tumor-bearing severe combined immunodeficient (SCID) mice after injecting each 111In-labeled radiopeptide, with or without PA co-administration.
Results: The presence of PA significantly increased the amount of intact [111In-DOTA-Ala1]SS14 in mouse circulation at 5 minutes post-injection, rising dramatically from less than 2% to 86%. Concurrently, uptake in AR4-2J xenografts increased from under 1 percentage injected dose per gram of tissue (%ID/g) to 14 %ID/g at 4 hours post-injection. Similarly, PA co-administration led to a notable elevation in circulating intact 111In-PanSB1, from 12% to 80% at 5 minutes, and radiopeptide uptake in human PC-3 xenografts in SCID mice surged from less than 4 %ID/g to over 21 %ID/g at 4 hours. Consistent results were observed with [111In-DOTA]MG11; PA co-administration increased intact levels in the bloodstream from less than 5% to 70% at 5 minutes, leading to a substantial increase in radiotracer uptake in both AR4-2J tumors and A431(CCKR+) tumors (induced by A431 cells transfected to express human cholecystokinin subtype 2 receptor) in mice at 4 hours, increasing from 2 %ID/g to over 15 %ID/g. SPECT/CT imaging of AR4-2J tumor-bearing mice at 4 hours effectively visualized this effect.
Conclusion: This study robustly demonstrates that co-administering key enzyme inhibitors can effectively prolong the survival of radiolabeled peptides in circulation, ensuring their protected transit to the intended tumor targets. This strategy notably amplified radiotracer accumulation in tumor xenografts in mice, potentially enhancing diagnostic sensitivity and therapeutic efficacy of radiopeptide drugs for cancer patients. Our findings offer compelling new avenues for utilizing biodegradable (radio)peptide drugs, whether of natural or synthetic origin, and provide a rationale for designing analogs with improved in vivo stability, ultimately to better serve and protect patients through improved cancer management.
The unique characteristic of peptide receptors being overexpressed on cancer cell membranes, compared to lower expression levels in adjacent healthy tissues, has created a valuable opportunity for visualizing and treating malignant tumors in cancer patients using radiolabeled peptide analogs, known as radiopeptides (1–3). These radiopeptide drugs are meticulously designed to deliver a chosen radionuclide specifically to their receptor targets located at primary and metastatic tumor sites. Diagnostic radionuclides emit γ photons, which are detected by external imaging devices like SPECT or PET cameras, enabling highly specific visualization of tumor lesions. Therapeutic radionuclides, on the other hand, emit particle radiation capable of inducing tumor cell apoptosis and death (4–6). The clinical implementation of this approach has seen success with [111In-diethylenetriaminepentaacetic acid (DTPA)]octreotide for diagnosing somatostatin receptor–positive neuroendocrine tumors (7). Further advancements, such as [177Lu-DOTA0,Tyr3]octreotate, are being investigated in phase 3 clinical trials for somatostatin receptor–targeted neuroendocrine tumor therapy (8,9). Moreover, peptide receptors that are abundantly expressed in other cancer types are emerging as potential targets for newly developed radiopeptide ligands (3).
The success of radiopeptide-based strategies hinges on the safe and effective delivery of intact radiopeptides to their receptor targets after intravenous administration. However, upon entering the circulation, radiopeptide integrity and target delivery are immediately challenged by ubiquitous proteolytic enzymes present in the blood and attached to the surface of blood cells and vascular walls. As radiopeptides circulate through major organs such as the liver, lungs, kidneys, and gastrointestinal tract, they encounter even more enzymes abundant in these compartments. The intricate relationships between native peptide ligands, their receptors, and the endogenous enzymes that regulate their interactions have long been recognized in both healthy and cancerous conditions. In the pursuit of peptide analogs resistant to enzymatic degradation, various structural modifications have been explored, including amino acid substitutions, reduction or methylation of biodegradable bonds, cyclization, and multimerization (10,11). While these approaches can yield more stable analogs, they are often costly, time-consuming, and may result in analogs with suboptimal pharmacologic properties.
Instead, we propose exploring the untapped potential of enzyme inhibitors to optimize radiopeptide delivery to tumor-associated receptor targets. Our aim is to enhance diagnostic sensitivity and improve therapeutic efficacy against both primary and disseminated cancer. By co-injecting a single inhibitor of neutral endopeptidase (NEP; EC 3.4.24.11; neprilysin; CD10) (12) into experimental animals, we successfully induced significant increases in the levels of intact circulating radiopeptide analogs from the somatostatin, gastrin, and bombesin families. Crucially, and to our knowledge for the first time, we achieved a notable amplification of radiopeptide uptake in various tumor xenografts in mice that express the corresponding receptor targets. This strategy allows the enzyme inhibitors to serve and protect the therapeutic radiopeptides, ensuring they reach their intended destination.
Specifically, we intravenously administered phosphoramidon (PA) alongside different radiopeptides in mice. PA is a potent, reversible, and competitive NEP inhibitor, and its binding structure at the active site of human NEP has been well-defined (13). Due to its high water solubility, PA can be co-injected as a bolus with a radiopeptide in doses that effectively inhibit NEP in vivo. Initially isolated from Streptomyces tanashiensis cultures, convenient synthesis methods for PA have recently become available (14,15).
This study included three types of 111In-labeled and DOTA-modified radioligands representing the somatostatin-14, bombesin, and gastrin peptide families. First, we examined the effect of PA co-injection on the stability and localization of [111In-DOTA-Ala1]SS14 (111In-DOTA-Ala-Gly-c[Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-Ser-Cys]-OH) in mice bearing somatostatin subtype 2 receptor (sst2)–expressing AR4-2J tumors (16). Our second example involved the bombesin analog 111In-PanSB1 ([111In-DOTA-PEG2-dTyr-Gln-Trp-Ala-Val-βAla-His-Phe-Nle-NH2), based on a previously reported sequence with a pan–bombesin receptor binding profile (17–19). We assessed the effect of PA on in vivo stability and tumor uptake in human PC-3 xenografts expressing the gastrin-releasing peptide receptor (GRPR) in mice (20,21). Our third example focused on the gastrin–cholecystokinin peptide family, evaluating the impact of PA co-injection on the in vivo stability of [111In-DOTA]MG11 (111In-DOTA-dGlu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2) and its uptake in two mouse tumor models: a double A431-CCK2R(+) and A431-CCK2R(−) xenograft model (induced by A431 cells transfected to express the human cholecystokinin subtype 2 receptor [CCK2R] and A431 cells lacking CCK2R expression, respectively), and the CCK2R-expressing AR4-2J tumor model (22–24). [111In-DOTA]MG11 had been previously reported to exhibit favorable low renal accumulation, but compared to full-chain gastrin analogs, it showed lower metabolic stability and reduced tumor targeting (23,25).
The findings of this study highlight a significant role for NEP in the in vivo processing of N-terminal radiometallated peptides, which is consistent with NEP’s widespread and abundant presence in the human body (12). The importance of NEP in the catabolism of the radiopeptides tested and potentially other classes of radiopeptide ligands warrants further investigation. We believe this preliminary study will encourage future research to elucidate the key peptidases involved in the breakdown of intravenously administered (radio)peptide drugs.
MATERIALS AND METHODS
Materials and Instrumentation
All chemicals used were of reagent grade. Peptides were synthesized or purchased from commercial sources. [DOTA-Ala1]SS14, [Tyr3]octreotate (H-dPhe-c[Cys-Tyr-dTrp-Lys-Thr-Cys]-Thr-OH), and demogastrin 2 [N4-Gly-dGlu-(Glu)5-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2] were synthesized on solid support as previously described (16,24). PanSB1 and DOTA-MG11 were obtained from PiChem, and [Tyr4]bombesin (Pyr-Gln-Arg-Tyr-Gly-Asn-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2) was purchased from Bachem AG. PA [*N*-(α-rhamnopyranosyloxyhydroxyphosphinyl)-l-leucyl-l-tryptophan × 2Na × 2H2O] was acquired from PeptaNova GmbH. For 111In labeling, 111InCl3 in 50 mM HCl at an activity concentration of 370 MBq/mL on the calibration date was purchased from Mallinckrodt Medical BV.
Radiochemical high-performance liquid chromatography (HPLC) analyses were performed using a Waters chromatograph equipped with a model 600 solvent delivery system, coupled with both a model 996 photodiode-array UV detector (Waters) and a Gabi γ detector (Raytest RSM Analytische Instrumente GmbH). Millennium Software (Waters) controlled the HPLC system and processed data. Radioactivity in samples was measured using an automatic well-type γ counter (NaI(Tl) 3′′-crystal; Canberra Packard Auto-Gamma 5000 series instrument). SPECT/CT images were acquired with a 4-head multipinhole NanoSPECT/CT camera (Bioscan Inc.) at the Animal Imaging Facility at Erasmus MC.
111In Labeling and Quality Control
Labeling with 111In was conducted by adding 10 nmol of peptide analog per 37–74 MBq of 111InCl3 in 0.1 M sodium acetate buffer and 10 mM sodium ascorbate. The typical end pH was 4.6. Labeling was completed after 20 minutes of incubation in a boiling water bath (26). Prior to HPLC quality control, ethylenediaminetetraacetic acid in 0.1 M acetate buffer was added to the labeling reaction mixture at a final concentration of 1 mM as a scavenger for free 111In3+ (27). For DOTA-MG11, Met (5 μL of a 0.1 M solution) was added to prevent oxidation of the Met residue to the corresponding sulfoxide (28).
Radiochemical analyses were performed using an XBridge Shield RP18 column (5 μm; 4.6 × 150 mm; Waters) with a matching 2-cm guard column. Elution was achieved with a linear gradient of 0.1% trifluoroacetic acid–water solution and acetonitrile (starting at 10% acetonitrile, increasing by 1% per minute) at a flow rate of 1 mL/min.
Cell Cultures
The rat pancreatic tumor cell line AR4-2J, endogenously expressing sst2 and rat gastrin/CCK2R, was kindly provided by Stephen Mather (St. Bartholomew’s Hospital, London, U.K.) and cultured as previously described (29,30). Human androgen–independent prostate adenocarcinoma PC-3 cells (LGC Promochem), endogenously expressing human GRPR (20), were cultured in Dulbecco modified Eagle medium with GlutaMAX-I (Gibco BRL Life Technologies), supplemented with 10% (v/v) heat-inactivated fetal bovine serum, penicillin at 100 U/mL, and streptomycin at 100 μg/mL. The human epidermoid carcinoma cell line A431, transfected to stably express the human CCK2R [A431-CCK2R(+)] or lacking CCK2R expression [A431-CCK2R(−)], was a generous gift from Otto C. Boerman (Department of Nuclear Medicine, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands) and Luigi Aloj (Istituto di Biostrutture e Bioimmagini, Consiglio Nazionale delle Ricerche, Naples, Italy). Transfected cells were cultured in Dulbecco modified Eagle medium with GlutaMAX-I, supplemented as above, but with G418 (Geneticin; Gibco BRL Life Technologies; 250 μg/mL) added to the medium (22). All culture reagents were obtained from Gibco BRL Life Technologies or Biochrom KG Seromed. Cells were maintained in a humidified atmosphere with 5% CO2 at 37°C. Cell confluence was monitored, and passages (1:3 or 1:4) were performed every 2–5 days using a 0.05% trypsin–0.02% ethylenediaminetetraacetic acid (w/v) solution.
Metabolic Studies in Mice
A bolus of 111In-labeled radiopeptide solution (100 μL; 11–22 MBq; 3 nmol of total peptide in physiologic saline–ethanol [9:1, v/v]), along with vehicle (100 μL of physiologic saline–ethanol [9:1, v/v]) (control group) or PA (100 μL of vehicle containing 300 μg of PA) (PA-treated group), was injected into the tail veins of healthy Swiss albino mice (National Center for Scientific Research “Demokritos” Animal House). Five minutes post-injection, blood (0.5–1 mL) was collected from the hearts of the animals and immediately transferred to prechilled polypropylene tubes containing ethylenediaminetetraacetic acid, and placed on ice. For [111In-DOTA]MG11, tubes also contained Met (final concentration of 1 mM) to prevent Met residue oxidation. Blood samples were centrifuged (10 min, 2,000*g*, 4°C; Hettich Universal 320R centrifuge), plasma was collected and mixed 1:1 (v/v) with chilled acetonitrile, and samples were centrifuged again (10 min, 15,000*g*, 4°C). Supernatants were concentrated to a small volume under gentle N2 flux at 40°C, diluted with physiologic saline (≈400 μL), and filtered through a Millex GV filter (0.22 μm).
Aliquots were analyzed by reversed-phase HPLC using a Symmetry Shield RP18 column (5 μm; 3.9 × 20 mm; Waters). Elution was performed over 50 minutes at 1 mL/min with a gradient from 100% A and 0% B to 50% A and 50% B, where A was 0.1% trifluoroacetic acid in H2O and B was acetonitrile. Elution times of intact radiotracers were determined by co-injecting the respective 111In-labeled radiopeptide.
Biodistribution of 111In-Labeled Radioligands in Xenograft-Bearing severe combined immunodeficient (SCID) Mice
Six-week-old SCID mice upon arrival (National Center for Scientific Research “Demokritos” Animal House) were inoculated in their flanks with a bolus (≈150 μL) containing a suspension of 1 × 107–2 × 107 freshly harvested cells in sterile physiologic saline. Animals were maintained under aseptic conditions until palpable tumors developed at the inoculation site. Palpable tumor development took 12 days for AR4-2J (350 ± 20 mg [mean ± SD]), 3 weeks for PC-3 (150 ± 30 mg), and 6 days for A431-CCKR(+) or A431-CCKR(−) tumors (260 ± 80 mg).
On the experiment day, a bolus of 111In-labeled radiopeptide solution (100 μL; 37–74 kBq; 10 pmol of total peptide in vehicle), with vehicle (100 μL) (control group), PA (100 μL of vehicle containing 300 μg of PA) (PA-treated group), or PA plus a receptor blocker (100 μL of vehicle containing 300 μg of PA and excess receptor blocker) (in vivo receptor blockade group), was injected into the tail veins of mice. For [111In-DOTA]MG11, Met at a final concentration of 1 mM was also included. For in vivo receptor blockade, animals were co-injected with PA and 40 nmol of [Tyr3]octreotate (sst2 blockade), 40 nmol of [Tyr4]bombesin (GRPR blockade), or 40 nmol of demogastrin 2 (CCK2R blockade). Animals were randomly assigned to each group. Additionally, two AR4-2J tumor–bearing animal groups were injected with [111In-DTPA]octreotide with either vehicle (100 μL) (control group) or PA (100 μL of vehicle containing 300 μg of PA) (PA-treated group).
Animals were euthanized in groups of 4 at 4 hours post-injection. Samples of blood, urine, and organs of interest were collected, weighed, and radioactivity was measured using an automatic well-type γ counter. Data were calculated as percentage injected dose per gram of tissue (%ID/g) using standard solutions and Microsoft Excel. Results are presented as mean ± SD, calculated using PRISM 2.01 (GraphPad) software.
Statistical analysis using the unpaired 2-tailed Student t test was used to compare control, PA-treated, and in vivo receptor blockade animal groups at 4 hours post-injection. P values less than 0.05 were considered statistically significant.
SPECT/CT Imaging of [111In-DOTA]MG11 in AR4-2J Tumor–Bearing Nude Mice
NMRI nu/nu mice (body weight ≈30 g; Harlan CPB) were inoculated subcutaneously in the shoulder region with 106 AR4-2J cells (injection volume 200 μL). Imaging commenced when tumors reached approximately 1 cm in diameter. Animals were anesthetized with isoflurane (Nicholas Pyramal Ltd.) and body temperature was maintained with a heated bed during imaging. Post-examination, animals were euthanized.
All animals received [111In-DOTA]MG11 (45 MBq; 0.5 nmol), with either vehicle or PA (300 μg), via tail vein injection 4 hours prior to SPECT imaging (total injection volume 200 μL). SPECT/CT images were acquired using a 4-head multipinhole SPECT/CT camera with energy peak settings at 171 and 245 keV (window width ± 10%) for 111In. Nine-pinhole apertures (diameter 1.4 mm) were used on each camera head (detector 230 × 215 mm). The matrix was 256 × 256. Animals were positioned at the center of rotation, with a fixed radius of rotation. SPECT images were acquired with 20 projections, 60 seconds per projection, and a quality factor of 0.8. Images were reconstructed using the ordered-subset expectation maximization method with HiSPECT NG software (Scivis Wissenschaftliche Bildverarbeitung GmbH), 24 iterations, and a voxel size of 0.3 × 0.3 × 0.3 mm. CT images were acquired with 180 projections, 45-kVp tube voltage, 500-ms exposure time, and a voxel size of 0.2 × 0.2 × 0.2 mm. Reconstruction was performed using the exact-cone-beam filtered backprojection algorithm. SPECT images were resampled to CT resolution and registered to CT images using VivoQuant program (version 1.22; inviCRO LLC).
All animal experiments were conducted in accordance with European and national regulations and after protocol approval by national authorities.
RESULTS
111In-Labeled Radiotracers
Incorporation of 111In into the DOTA chelator coupled to the three peptide analogs was achieved by heating at 90°C for 20 minutes in an acidic medium, consistent with prior research (26). Reversed-phase HPLC analysis of labeling reaction mixtures showed that all three 111In-labeled radiotracers eluted as a single radiochemical species with >95% purity.
Effect of PA on In Vivo Stability and AR4-2J Tumor Uptake of [111In-DOTA-Ala1]SS14 in Mice
The impact of PA co-injection on the stability and tumor localization of [111In-DOTA-Ala1]SS14 in mouse models is depicted in Figure 1. Analysis of blood samples 5 minutes after [111In-DOTA-Ala1]SS14 injection into healthy mice revealed nearly complete radiopeptide degradation (Fig. 1A) (16,31). However, with PA co-injection, the amount of parent radiopeptide in the blood increased to 86% (Fig. 1B). Importantly, this enhanced circulating intact radiotracer translated to a significant amplification of radiotracer levels in AR4-2J tumors at 4 hours post-injection in SCID mice. Specifically, uptake in sst2-expressing tumors rose from under 1 %ID/g to 14 %ID/g (Fig. 1C and inset). Conversely, tumor uptake decreased to less than 0.3 %ID/g after co-injection of both PA and excess [Tyr3]octreotate, indicating an sst2-mediated process. In contrast, at 4 hours post-injection of metabolically stable [111In-DTPA]octreotide, uptake in AR4-2J tumors (n = 7) was 5.5 ± 1.0 %ID/g in the control group and 5.6 ± 1.0 %ID/g in the PA-treated group.
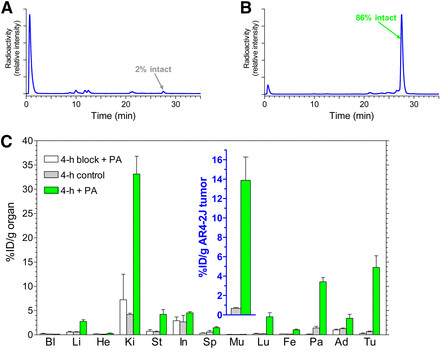 FIGURE 1.
FIGURE 1.
Effect of PA on In Vivo Stability and Human PC-3 Xenograft Uptake of 111In-PanSB1 in Mice
Figure 2A demonstrates the rapid breakdown of 111In-PanSB1 within 5 minutes of entering the bloodstream in healthy mice. However, co-administration of PA resulted in a remarkable increase in circulating 111In-PanSB1 levels, from 12% to 80% (Fig. 2B). Consistent with this, radioactivity uptake in human GRPR–positive PC-3 xenografts in SCID mice escalated from less than 4 %ID/g to over 21 %ID/g at 4 hours after radiopeptide co-injection with PA (Fig. 2C and inset). Uptake in human xenografts was reduced to below 0.9 %ID/g by co-injection of both PA and excess [Tyr4]bombesin, confirming GRPR specificity.
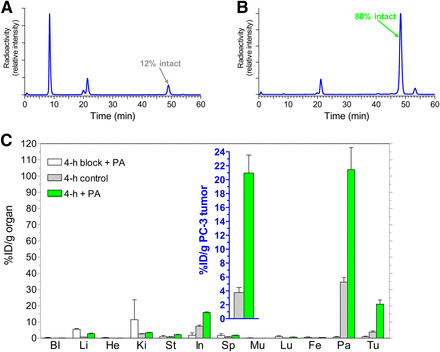 FIGURE 2.
FIGURE 2.
Effect of PA on In Vivo Stability and A431-CCK2R(+) Tumor Uptake of [111In-DOTA]MG11 in Mice
HPLC analysis of blood samples 5 minutes post-injection of [111In-DOTA]MG11 showed only 5% intact radiopeptide in mouse blood (Fig. 3A). PA treatment significantly increased circulating [111In-DOTA]MG11 levels to 70% (Fig. 3B). The impact on tumor uptake was studied using a double-tumor model in SCID mice (23). PA co-injection strikingly increased uptake, from 2 %ID/g to over 15 %ID/g, specifically in A431-CCK2R(+) tumors, not A431-CCK2R(−) tumors (Fig. 3C and inset). CCK2R-negative tumors exhibited minimal uptake (<0.5 %ID/g).
 FIGURE 3.
FIGURE 3.
Effect of PA on AR4-2J Tumor Uptake of [111In-DOTA]MG11 in Mice: Visualization with SPECT/CT
PA treatment markedly increased [111In-DOTA]MG11 uptake in AR4-2J tumors (endogenously expressing rat CCK2R), rising from under 2 %ID/g to over 16 %ID/g at 4 hours post-injection. After co-injection of PA and excess demogastrin 2 (24), tumor uptake decreased to 0.3 %ID/g, indicating CCK2R-mediated accumulation (Fig. 4A and inset). SPECT/CT small-animal imaging of AR4-2J tumor–bearing mice confirmed these findings, demonstrating significantly improved tumor visualization with PA (Figs. 4B and 4C).
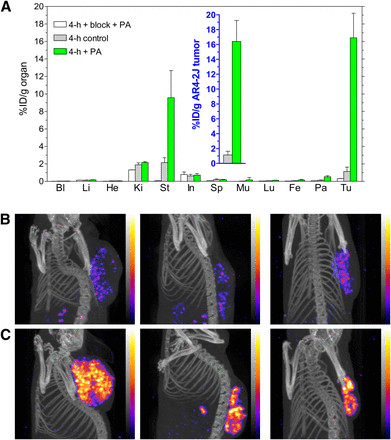 FIGURE 4.
FIGURE 4.
DISCUSSION
The past two decades have witnessed remarkable progress in experimental and clinical oncology, including the recognition of peptide receptor overexpression on cancer cell surfaces, exceeding levels in surrounding healthy tissues. Peptide receptors, belonging to the serpentine G protein–coupled receptor (GPCR) superfamily, are increasingly recognized for their role in cancer (1–3). Nuclear medicine researchers have leveraged these advances, developing new molecular tools based on radiolabeled peptide ligands to locate and combat primary and metastatic disease. Radiopeptides are designed to carry radionuclides suitable for SPECT (γ emitters), PET (positron emitters) imaging, or radionuclide therapy (α, β, or Auger electron emitters) to GPCR targets with high lesion specificity (4,6).
The clinical application of radiolabeled somatostatin analogs in diagnosing, staging, and treating neuroendocrine tumors overexpressing sst2 is particularly relevant. The success of this strategy largely depends on the high metabolic stability of the radiopeptides used. Unlike rapidly biodegradable native somatostatin-14 (31), sst2-directed radiopeptide drugs (e.g., [111In-DTPA]octreotide) are based on cyclic octapeptide analogs like octreotide, which exhibit robust in vivo stability (7–9). While neuroendocrine tumors are relatively rare, other GPCR targets for radiopeptide ligands are abundantly expressed in more prevalent human cancers (3). For example, GRPRs are highly expressed in prostate, breast, and lung cancers, making them potential targets for bombesin-like radioligands (32,33). Expanding the clinical success of [111In-DTPA]octreotide to other radiopeptide families could significantly broaden the impact of radiopeptides in oncology.
However, progress in this area is limited, partially due to the poor metabolic stability of radiopeptide drugs. Structural modifications aimed at improving metabolic stability often compromise receptor affinity or pharmacokinetic profiles (10,11). Furthermore, common in vitro assays used to assess metabolic stability, such as incubation in mouse or human serum, neglect the enzymatic activity of vasculature walls, blood cells, and major organs, potentially overestimating in vivo stability (34).
This study explored a novel approach to enhance tumor targeting: in vivo co-administration of an enzyme inhibitor and a radiopeptide. The inhibitor–radiopeptide pair is chosen to ensure the radiopeptide is protected from degradation immediately upon entering circulation until it reaches its tumor-associated receptor target. Transient inhibition is sufficient to safeguard radiopeptide transit to the target. We hypothesize that increased tumor uptake through this strategy will translate to improved diagnostic sensitivity and therapeutic efficacy of radiopeptide drugs for cancer patients. This is a strategy designed to serve and protect the efficacy of these vital diagnostic and therapeutic agents.
We demonstrated the feasibility of this approach using radiopeptides from the somatostatin, bombesin, and gastrin families. Co-injection of PA, a single NEP inhibitor (12,13), with test radiopeptides, resulted in a marked increase in tumor uptake in mouse models, correlated with improved circulating levels of intact drug. Significantly, PA had no measurable effect on the tumor uptake of metabolically stable [111In-DTPA]octreotide in the same sst2-positive model used for [111In-DOTA-Ala1]SS14. This supports our hypothesis that increased tumor uptake resulted directly from enhanced radiopeptide bioavailability due to NEP inhibition by PA. The profound impact of a single NEP inhibitor on the bioavailability and tumor targeting of tested radiopeptides is notable, considering the vast number of proteases in the human body. The human degradome includes at least 569 proteases and homologs across five classes (35,36). While NEP appears crucial in initiating the breakdown of the tested radiopeptides, further research is needed to identify other potential contributing enzymes.
The proposed strategy holds significant promise for biodegradable radiopeptides, including those based on native sequences (16,31, which are evolutionarily optimized for receptor interaction. This approach could broaden the application of radiopeptide analogs to more cancer types. It also provides a versatile method for identifying enzymes responsible for in vivo degradation of specific radiopeptide drugs. This information is vital for rationally designing stabilized radiopeptide analogs and may also benefit other peptide drugs, such as peptide-conjugated cytotoxic drugs, optical imaging probes, and phage display-selected peptidic ligands (37).
CONCLUSION
The findings of this study underscore the critical role of radiopeptide in vivo stability for effective tumor targeting in cancer imaging and therapy. Unexpectedly, our results indicate that a single peptidase (NEP) is a key factor in the rapid in vivo breakdown of intravenously administered radiopeptides from at least the somatostatin, bombesin, and gastrin families.
Importantly, our findings offer exciting opportunities to serve and protect biodegradable radiopeptides, enhancing their delivery and accumulation at tumor sites by co-injecting a protease inhibitor like PA. This amplification of radiotracer levels in tumor lesions is expected to significantly improve diagnostic sensitivity and therapeutic efficacy. The extensive existing knowledge of peptidase inhibitors, including some tested in human studies or already approved drugs, highlights the translational potential of this strategy for clinical practice.
The novel concept of radiopeptide drug–enzyme inhibitor co-injection to optimize tumor uptake is particularly valuable for molecular imaging and therapy protocols. Nuclear medicine protocols, involving single or limited systemic drug administrations, require only short-term enzyme inhibition. Protection is primarily needed between intravenous radiopharmaceutical injection and tumor target delivery—a brief period for small, rapidly localizing radiopeptide ligands. Water-soluble agents that “escort” radiopeptides through hydrophilic body compartments and induce fast, transient enzyme inhibition offer an elegant solution for optimizing tumor targeting, effectively working To Serve And Protect the patient through improved drug delivery.
DISCLOSURE
The publication costs for this article were partially defrayed by page charges, hence it is marked “advertisement” as per 18 USC section 1734. No potential conflicts of interest were reported.
Acknowledgments
We thank Aikaterini Tatsi, Panteleimon J. Marsouvanidis, and Aikaterini Kaloudi for their assistance in metabolic and biodistribution experiments, and Saskia Berndsen, Stuart Koelewijn, Jan de Swart, and Harald Groen for expert support during the SPECT/CT study.
Footnotes
- Published online Nov. 28, 2013.
- © 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
[List of references as in the original article]
- Received for publication July 15, 2013.
- Accepted for publication September 25, 2013.
